















- Locations
- United States
- US Blogs
- Making All the Right Moves When Resources are Tight
Organizational maturity and best practices for advocacy groups to prioritize time and investment resources is the focus of this blog based on the IQVIA Institute’s 2021 Patient Advocacy Summit, held on December 1, 2021. An earlier blog focused on community building and mobilization by patient advocacy groups.
The journey from the ‘kitchen table’—where many patient advocacy groups originate—towards treatments (and ultimately a cure) is the topic of this blog, based on the experience of two advocacy organizations. Aimed primarily at newer organizations, this Summit session examined competing priorities, which range from establishing a formal diagnosis and identifying standards of care to building research networks and care centers of excellence. All these must be achieved while managing the advocacy group’s community outreach and engagement, data governance, and legal considerations.
The organizational maturity focus group included the Friedreich’s Ataxia Research Alliance, the Primary Ciliary Dyskinesia (PCD) Foundation, and experts from IQVIA. Friedreich's ataxia is a life-threatening, inherited neuromuscular disorder affecting vision, hearing, speech, strength, and coordination; primary ciliary dyskinesia is an umbrella term for inherited disorders of microscopic, whip-like organelles called cilia that line the respiratory tract, eustachian tubes of the ear, reproductive organs, and ventricles of the brain.
The Primary Ciliary Dyskinesia Foundation
The Primary Ciliary Dyskinesia (PCD) Foundation (https://pcdfoundation.org) was established in 2001 by a parent, Michele Manion, and a patient, Lynn Ehrne, in 2001. The primary purpose for starting the foundation was to address severe unmet needs in the PCD patient community, including diagnostic challenges, lack of evidence to support therapies, inadequate demographic information and paucity of data related to the natural history of this disorder.
Key learnings offered by PCD Foundation leadership include the importance of not being intimidated about setting up an advocacy group, and the value of collaborating with all stakeholders in the disease area. An important step to prepare for partnerships with industry is to build assets and tools that will support future trial. Finally, they emphasized the importance of staying hopeful.
Milestones in the organization’s history include:
- In 2002, the PCD Foundation partnered with the University of North Carolina at Chapel Hill, receiving a five-year grant to establish the NIH-sponsored Genetic Disorders of Mucociliary Clearance Consortium (GDMCC); this was funded again in 2019 for years 15-20. The multi‐center model created by the GDMCC formed the basis for the PCD Foundation Clinical and Research Centers Network program, which now has 40+ sites in the U.S. and Canada.
- Funding was used to advance natural history studies, improved diagnostic capabilities, identifying disease-related genes and associated disease severity, longitudinal studies, identifying therapeutic targets and endpoints for trials. The Path to Clinical Trials Program was launched in 2011 and is still ongoing; one drug is in development.
- The PCD Foundation Registry (Discovery Ecosystem) was established through a 2019 pilot, involving a five-site natural history study; as of 2022, there are 10-25+ sites
- In November 2021, the PCD Foundation joined the Chan Zuckerberg Initiative, the Rare As One Network, receiving a grant for research to enhance health outcomes and improve access to genetic testing for PCD and related conditions in underserved areas across the Americas. Expanded gene identification is a critical step to improve early diagnosis.
- Progress through committed, collaborative partnerships is key; relationships are in place with a wide range of institutions and labs, professional organizations, pharmaceutical companies, and patient advocacy groups.
- The group is now working to scale its clinical and research centers network and build an innovative technology platform to accelerate discoveries and new therapies, with the ultimate goal of finding a cure for PCD. The Foundation is also starting to look at options for some decentralized clinical trial elements, to improve access for patients who live far away from brick-and-mortar clinical sites. Examples might include validated instruments that patients could use at home.
The Friedreich’s Ataxia Research Alliance (FARA)
At a more advanced stage in organizational development is the Friedreich’s Ataxia Research Alliance (https://www.curefa.org). This was founded by Ron and Raychel Bartek in 1998 after their son, Keith, was diagnosed with this disease. At that time, there was no treatment, little research, no pharmaceutical industry involvement, and no clinical trials.
FARA’s advances have built on the foundation of the discovery of the gene involved in Friedreich’s ataxia. With a workshop grant from the National Institutes of Health (NIH), the group held the world's first scientific conference on this disease in 1999, co-hosting this three-day event at the NIH with the NIH Neurological Institute and assembling 80 scientists—15 from NIH and the remainder from around the world. The latest FARA international conference included more than 400 scientists. The initial conference launched the group’s hallmark collaborative culture, and the group also began awarding research grants at that point, starting with one for $1,000.
FARA’s goal was to grow the field with the trust and support of its patient and family community, tapping into a dedicated group of academic scientists for advances in basic and translational science, and eventually in clinical research. Early efforts focused on developing natural history data sets within a standardized digital platform, a contact registry, and evaluating potential clinical outcome measures. The scientists set out to develop the translational tools, including assays, biomarkers, cell and animal models, biorepositories and neuroimaging that would be needed to drive research and development. Three consortia, focusing on biomarkers, neuroimaging, and cell and animal models, are currently jointly funded by FARA and industry partners.
To date, FARA’s achievements include:
- Providing funds of around $70 million for research, and accessing a similar sum in collaborative funds
- Advancing an expanding R&D pipeline for Friedreich’s ataxia with multiple targets in small molecules and other therapeutic approaches aimed at the underlying cause of the disease, including protein supplementation (underway), gene therapy, and gene editing (Figure 1). One small molecule drug has completed its pivotal study, and the company is submitting its new drug application in the first quarter of 2022.
- Growing stakeholder inclusion, with a community of scientists that now numbers more than 400, about four dozen pharmaceutical company partners, and numerous advocacy partners, has enabled a total of around 15 clinical trials already conducted, taking place at a dozen sites in the U.S., Canada, Australia, New Zealand, Brazil, and India, along with collaborative sites in Europe. Nine additional clinical trials are currently under way.
- Developing a culture of global collaboration and unity among all stakeholders, including:
- Gaining the trust of highly dedicated, motivated, engaged families
- Building strong relationships with NIH, FDA, Congress, industry, and other advocacy groups.
- Forming scientific collaborations via FARA grants to characterize Friedreich’s ataxia, the disease pathology, and the mechanism of action of potential therapeutics. Efforts focus on:
- Translational tools (assays, cell/animal models, biorepositories, imaging)
- A global clinical network and a multi-language online contact registry, including genetic confirmation and some clinical details
- A natural history study, based on annual visits/exams for pediatric and adult patients
- Biomarker and neuro-imaging consortia, which are co-funded with industry partners in the pre-competitive space
Solid relationships with industry have been based on the fact that FARA has a turn-key clinical trials operation into which drugs can be inserted. FARA provides access to its natural history data and patient registry without charge; it receives industry donations, but no fees related to clinical trials. On occasions, trial cohorts of around 100 patients for a phase 3 trial have been successfully identified within hours by matching inclusion/exclusion criteria with known features of each patient. Educational symposia are held to keep patient families informed of research progress and upcoming trials. Options for virtual trials are also being explored by FARA. While none have been implemented to date, the group is working on digital platforms and wearable devices that could provide real-world-data from patients during their daily lives, in their homes.
Figure 1: The Friedreich’s Ataxia treatment pipeline
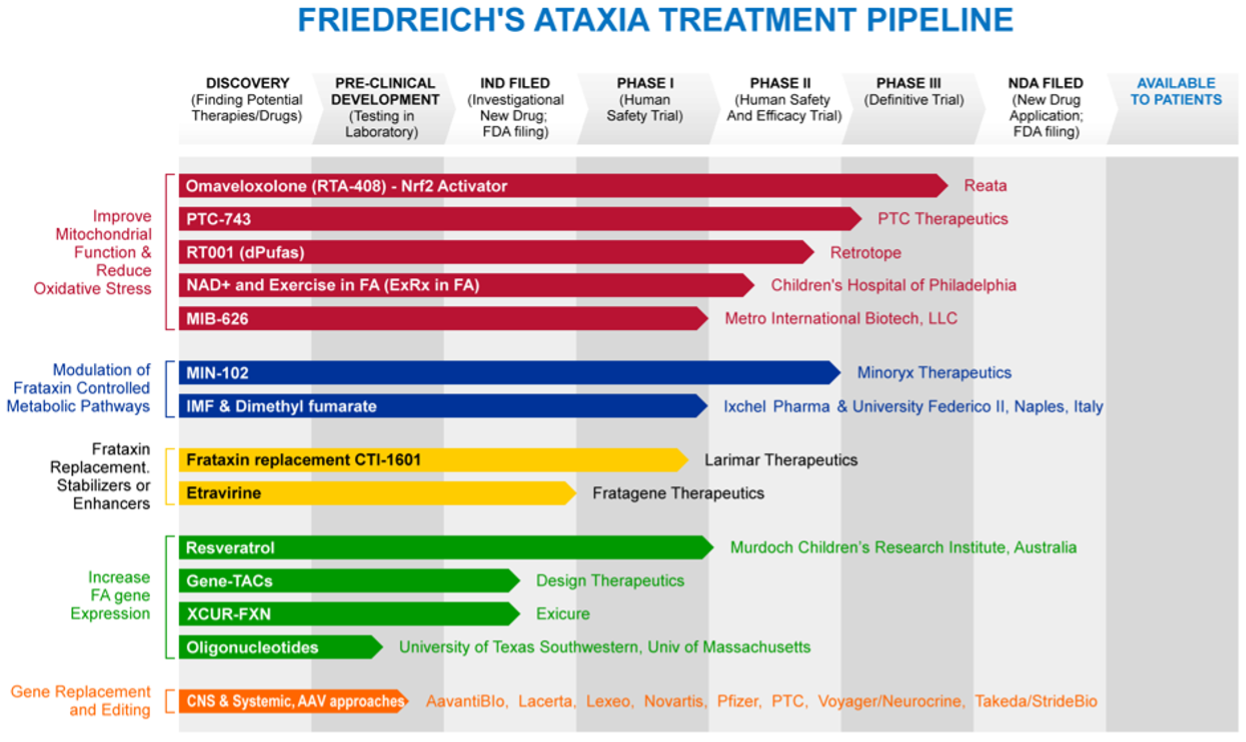
Overall, the experiences of both the PCD Foundation and FARA provided encouragement for other patient advocacy organizations. Each offered proof that it is possible to replace a sense of hopelessness and isolation with hope, confidence, and trust within an engaged community. This can build a sense of optimism that together, stakeholders drive progress towards effective treatments for their disease.
About the Summit
The 2021 Patient Advocacy Summit, organized by the IQVIA Institute in conjunction with IQVIA Healthcare Solutions, gathered leaders from across the patient advocacy community that share an interest in the role they play in advancing research into therapeutics and cures through the use of health data to understand patients, disease, optimal care, and improve outcomes. The Summit convened 150 advocacy community leaders from patient organizations to share ideas and solutions for issues such as diversity and inclusion in research and RWE, evolving policy and regulatory landscape, opportunities for driving their own research, and discussions around their unique ability to capture the patient voice.
Participants
Ron Bartek, President & Co-Founder, Friedreich’s Ataxia Research Alliance
Carey Kauffman, Patient Registry Director, PCD Foundation
William Lawrence, Senior Director, Registry Center of Excellence, IQVIA Healthcare Solutions
References
1 https://chanzuckerberg.com/rao/pcd-foundation/




