Outdated compliance practices aren't viable amid evolving market and regulatory changes. IQVIA’s award-winning, end-to-end quality management system streamlines the product value chain, accelerates market entry, safeguards companies and patient safety, and meets complex regulatory demands.
















- Locations
- United States
- US Blogs
- The Importance of a Next Generation QMS
Every day, life sciences organizations rely on their quality management system (QMS) to drive people and processes to improve product quality, minimize risk, ensure patient safety, and help maintain regulatory compliance. A next generation QMS delivers even greater value by taking an organization’s quality maturity to the next level, also ensuring your operation is efficient and inspection-ready.
Let’s look at how a next generation QMS can advance your quality maturity and examine the five key characteristics that enable the technology to optimize your quality system performance.
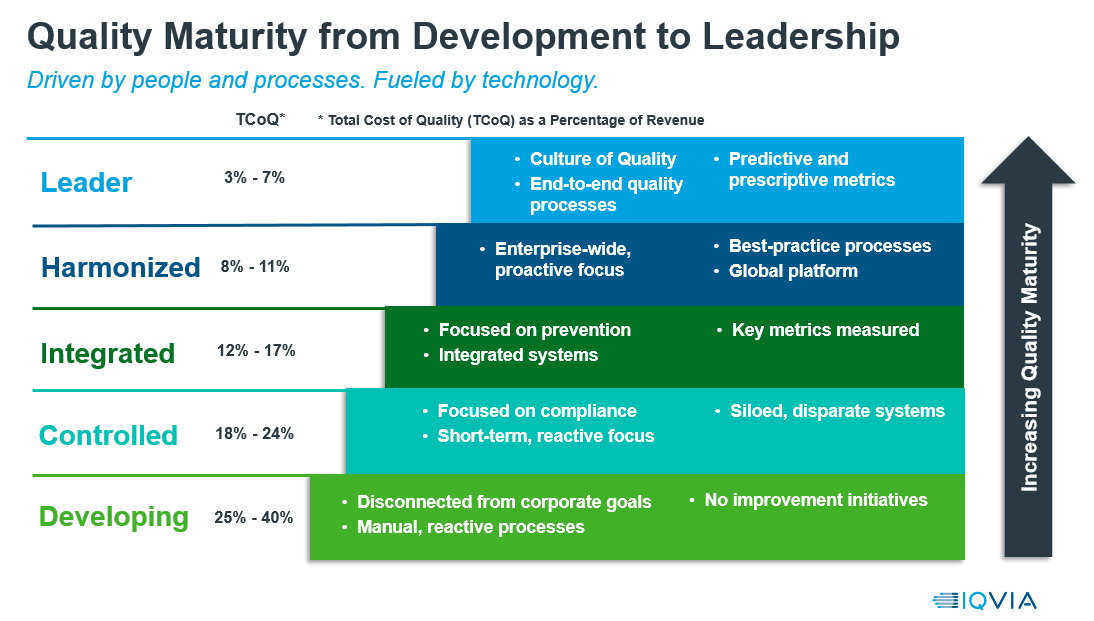
Advancing your quality maturity
Quality maturity can positively impact the total cost of quality. Those organizations that are further along on their quality maturity journey benefit from a lower cost of quality.
In the beginning stages of maturity, processes are manual and reactive. They’re mainly compliance-driven as opposed to quality improvement- driven. Many organizations in this stage are facing the challenges of GXP readiness and compliance, such as new and evolving regulations like EU MDR for the medical device industry.
As organizations increase in maturity, the focus moves to prevention, and the organization becomes better able to anticipate and manage risk. This can be driven and supported by integrated quality and IT systems. In higher stages of quality maturity, companies become more proactive and can harmonize processes across the business and even beyond their four walls to better manage supplier quality and other contracted activities.
At the highest level of maturity, organizations are operating within a true culture of quality, with end-to-end quality processes and the ability for metrics to help sustain and improve overall quality.
The goal of this journey is to achieve higher stages of maturity to gain the benefits of higher product quality, improved compliance, and reductions in overall cost of quality.
5 key characteristics of a next generation QMS
- Built for Life Sciences– With stringent regulatory requirements, it is critical that your QMS is compliance-ready and closed-looped for accuracy and efficiency. A purpose-built, integrated QMS should help eliminate siloed systems, drive harmonization, reduce overall cost of quality, and enable informed, data-driven decision making, while helping your organization adhere to industry regulations such as 21 CFR Part 11.
- Quality Intelligence and Reporting – Easy access to complete and accurate quality system data is critical for making informed, data-driven decision. Robust business intelligence (BI) combined with standard reports should provide instant feedback into quality trends, enabling your teams to make the right decisions at the right time. Personalized dashboards should allow a next generation QMS user to easily manipulate data and adjust their views so that they see the most relevant information for their role.
- Cloud – A highly secure, reliable, and efficient cloud environment will protect your quality system data in an industry-compliant environment. The cloud should scale as your business grows in order to accommodate your changing business needs.
- End-to-End EQMS Deployment and Optimization – In order to optimize your next generation QMS deployment, the solution provider needs deep life science expertise to aid your organization in your journey to process harmonization and the achievement of proactive quality.
- Intuitive and Modern User Interface (UI) - Having a consumer-grade user interface improves user adoption and allows for the inclusion of myriad users across the organization. The simplification of tasks improves user acceptance, utilization, and inclusion. An intuitive user interface can assist in shortening cycle times, reducing errors, and minimizing training needs. The user interface for a next generation QMS needs to be simple enough for the casual user, yet powerful enough for administrators and experts.
SmartSolve®, a Next Generation QMS
SmartSolve®, from IQVIA, helps organizations like yours maintain efficient and compliant quality management systems. SmartSolve’s integrated platform enables quality to become a centralized hub for continuous improvement throughout your business. For additional information about this next generation QMS, download the fact sheet.
Let’s talk! Schedule a personalized discovery session with one of IQVIA’s QMS solution experts today. We'll review your current quality processes and show you how to get the most out of them using SmartSolve.

IQVIA SmartSolve EQMS
IQVIA's integrated SmartSolve platform enables quality to become a centralized hub for continuous improvement throughout your business.
You May also be interested in
IQVIA Quality Compliance
Advancing Quality Maturity with People, Processes and Technology
Related solutions
Discover how AI and ML reduce risk and increase efficiency in adverse event reporting
From manufacturing oversight to regulatory requirements, manage quality across your organization with a single enterprise software solution.
Automate and standardize your regulatory management, from correspondence and commitments to registration and tracking.
IQVIA Vigilance Platform is our secure SaaS environment built to simplify safety and PV processes, while boosting speed, accuracy, and efficiency.
Harness the power of automation to execute streamlined end-to-end safety solutions while reducing costs.
Meet the challenge of changing stakeholder demands and increasing cost constraints with IQVIA's integrated technology services and analytics-driven offerings.




