The European Union (EU) Health Technology Assessment Regulation (HTAR) will come into effect on January 12th 2025, with the aim of reducing duplication across member states and ensuring more rapid access to innovative treatments. In addition to introducing joint clinical assessments (JCAs) for new medicines and medical devices, the HTAR also provides a framework for joint scientific consultations (JSCs), allowing health technology developers (HTDs) to obtain early advice on the evidence packages required for future JCAs and national HTA submissions.
The European Commission (EC) committee on Health Technology Assessment (HTA) published the draft of the implementing act (IA) for the Joint Scientific Consultation (JSC) for medicinal products on 1st October 2024, and the final IA will be adopted before the end of Q4 2024. The procedural guidance for JSCs will follow imminently, and future IAs will provide clarity on JSCs and JCAs of medical devices.
How will the JSC work?
JSC facilitates a discussion between HTDs and EU HTA bodies (HTAb) on clinical development programmes before the pivotal trial protocol lock. JSCs may also be conducted in parallel with scientific advice from the European Medicines Agency (EMA), where the timing and submission of briefing package to both bodies will be synchronised. The overall aim is to streamline regulatory and HTA advice procedures, thus optimising opportunities for the generation of robust evidence fit for both marketing authorisation applications and JCAs, particularly where there is a high unmet need and a lot of uncertainty.
The HTA Coordination group (HTACG) will set its request periods by 30th November of each year, in line with the adoption of its annual work plan. The HTACG must provide a minimum of two request periods each year. The number of slots is anticipated to be limited, with just ten slots available in 2025. HTDs will need to demonstrate that all of the JSC eligibility criteria are met; unmet medical need, first in class, potential impact on patients, public or healthcare systems, significant EU-cross border dimension, major union-wide added value and aligned with union clinical research priorities. Priority will be given to oncology and advanced therapeutic medicinal products and indications for which there is no established guidance for clinical development.
Following submission of the briefing package, the coordination group will share the ‘list of issues’ to be either addressed in writing before the meeting with HTAb or in the virtual meeting itself. Written response and any materials/ presentations to be used by the HTD in the meeting need to be submitted 10 days prior to the meeting.
JSC meetings will give HTDs the opportunity to discuss key concerns with a broad range of stakeholders, including JSC assessors, clinical and patient experts, as well as experts in the relevant disease or therapeutic area. If a JSC is held in parallel with EMA scientific advice, EMA representatives from the scientific advice working party will also participate in the discussions. Any updates on the development plan by the HTD can be reflected in the JSC advice if shared at least 10 days before the meeting.
Although timelines have not yet been clarified, it is anticipated to take approximately 4.5 months from the receipt of the draft briefing package, as per the current interim EMA/ HTA body parallel procedure that is being coordinated by Germany’s Federal Joint Committee (G-BA). Factoring in the need for alignment and development of the application at the start and alignment post-receipt of the final recommendations, this means that the decision to proceed with JSC needs to be made at least 10 months prior to pivotal trial protocol lock.
Considerations before applying for JSC
There are numerous asset- and company specific factors that need to be evaluated before making the strategic decision to apply for JSC. A risk-benefit assessment should be conducted to evaluate the pros and cons of seeking JSC or scientific advice in general. A ‘decision framework’ should involve cross-functional teams (e.g. market access, commercial, regulatory, medical, statistics, external engagement) factoring in all potential routes of advice (JSC, national advice procedures, advisory boards) and analyses of the specific objectives for seeking advice. Timing, eligibility, objectives of advice and budget are key factors to consider including in the decision framework. (Fig. 1).
Fig 1. Scientific advice strategic considerations
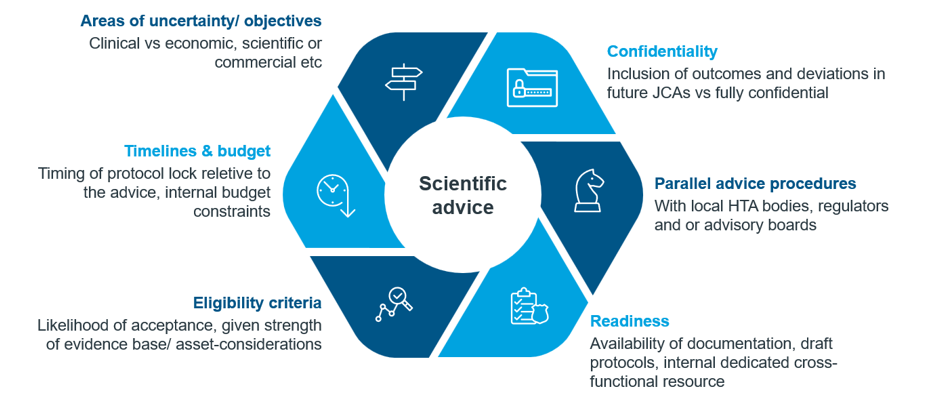
Confidentiality is another key factor to account for when considering advice options. Whilst not legally binding, HTDs will need to include the outcome of previous JSCs in JCA submissions, including justifications for deviations from the advice given. There may be some instances where it may not be practical or feasible to implement advice, which may be viewed unfavourably in local HTA procedures. The objectives of the advice and the specific questions included in the briefing package therefore need to be carefully considered.
A high bar for eligibility
As outlined previously, the eligibility criteria for JSC is focused on assets where there is a high unmet need and uncertainty (innovative products, with no or unsatisfactory standard of care). When applying for JSC, HTDs are therefore expected to clearly demonstrate how the product fulfils all (not some) of the stipulated criteria. Therefore, early phase work should focus on generating evidence that demonstrates fulfilment of these criteria to maximise chances of being selected for JSC. Given the anticipated high demand and limited number of slots, the bar will be set high for those seeking out the coveted slots.
Preparation is key to get the most out of the JSC meeting
HTDs are expected to drive the JSC meeting, focusing on the list of issues shared by the HTACG/ EMA. Depending on the number of issues identified, HTDs may not have sufficient time to discuss all of the issues. For this reason, it is important for HTDs to carefully consider the questions to be included in the briefing package to keep the discussion topics and recommendations manageable.
Attendees in the meeting will be limited; HTDs need to ensure that each participant they select has the necessary domain expertise to actively participate, summarising the issue(s), present the HTD’s position and address any feedback from the stakeholders. A mock meeting with all key stakeholders, to rehearse timing, presentation flow and anticipate potential questions from stakeholders are helpful to maximise the output of the meeting and ultimately, the final written advice.
Advice outcome – what’s next?
HTDs will receive the formal written advice separately from the JSC assessors and EMA approximately two weeks after the JSC meeting. HTDs should carefully review the advice and align cross-functionally on the modifications to the clinical trial design and evidence generation plans. As JSC advice needs to be disclosed in the JCA dossier, HTDs will need to consider the likely impact of deviations from the JCA recommendations and clearly justify rationale for not implementing advice to minimise risk. Similarly, any recommendations implemented based on the JSC advice should be documented and updated periodically as the clinical trial progresses.
Moving forward
Whilst not everything is yet fully clear, our experience with the JSC pilot shows that companies who carefully consider the need for JSC and meticulously plan for scientific advice will be able to better meet JCA requirements and thereby accelerate EU patient access.


























