Manage HCP consent to enable compliance with data privacy regulations.
Organizations are finding that integrating consent management technology is a strategic necessity. A unified approach is essential to manage consent effectively across all customer touchpoints, thus reducing significant compliance risks.
In a recent IQVIA webinar, “Navigating HCP Consent: Legislative Updates and Best Practices,” IQVIA experts reviewed legislative updates and effective strategies for managing healthcare professional (HCP) consent. During the session, two key polling questions to our audience provided valuable insights into current industry practices and concerns.
Biggest challenges in managing HCP consent
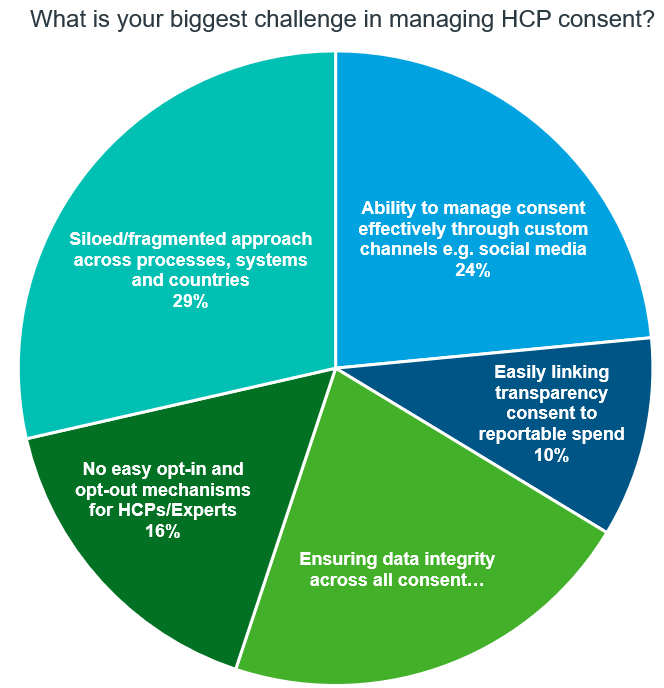
Source: IQVIA Commercial Compliance
Organizations face several challenges related to consent management, including fragmented consent across processes and systems, data integrity concerns, and the need to manage consent effectively through custom channels. These challenges pose significant compliance risks, especially given the GDPR and DPDPA mandates for an easy opt-out mechanism for healthcare professionals (HCPs).
Harnessing consent master technology can help overcome these complex challenges, not least by facilitating the handling of various consent purposes and channels to different HCP groups for more targeted engagement. Additionally, granting HCPs direct access to their consent and preference centers is essential for accurate consent status, building HCP trust and confidence. However, it should not be understated that implementing a consent master to harmonize and share consent data across the ecosystem enables organizations to drive a far greater reach of the target physicians, beyond the HCP collective only visited by the field teams, thus driving growth through the message expansion, while maintaining a balance with compliance and fulfilling data privacy requirements.
Capturing HCP consent
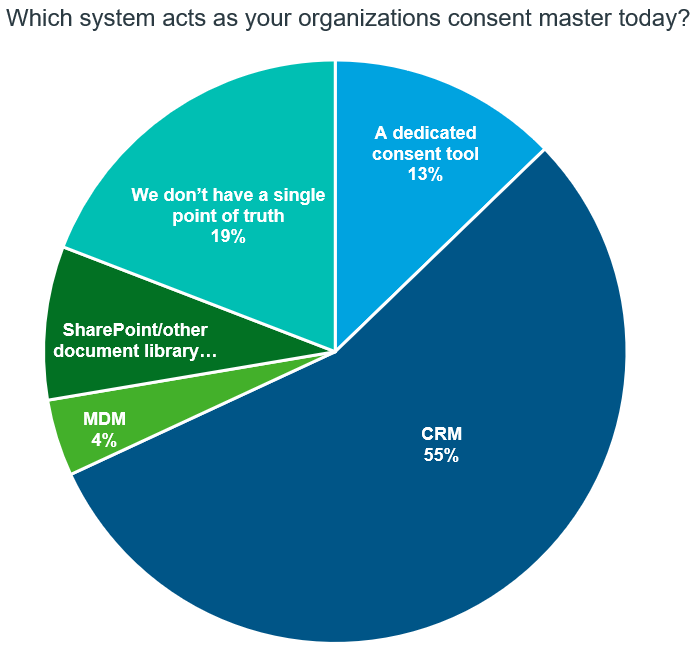
Source: IQVIA Commercial Compliance
According to the survey, 19% of respondents lack a single point of truth, while an additional 68% rely on systems or processes with limited functionality to serve as a consent master across their ecosystem. It is important to note the following:
- CRM (Customer Relationship Management) systems can be effective tools for capturing consent but in IQVIA’s experience these systems fall short in offering comprehensive consent management features and direct access for healthcare professionals (HCPs), which is a key requirement when it comes to giving data subjects the ability to manage their consent.
- Dispersed consent systems pose compliance risks if data is not consistently updated, consolidated, and shared across applications within the ecosystem.
- From an operational perspective, a dedicated consent management solution is also a crucial element for successful omnichannel marketing and communication campaigns, providing the ability to track and monitor opt-ins through the different channels and sources, while facilitating insights on the optimum approach for HCP group/specialty your campaigns.
- Managing consent is as important as capturing consent in the first instance. For continued trust and confidence from the HCP community, any consent updates, or opt-outs (unsubscribing consent) should be handled effectively across all platforms in the ecosystem, in the briefest period possible to avoid actions that do not respect the latest wishes of the HCP.
What other factors should be considered if you don’t have a dedicated consent tool or single source of truth?
- It is highly recommended to have a consent repository or master that utilizes a unique external identifier (such as OneKey ID, CIAM (Customer Identity and Access Management) ID or MDM (Master Data Management) ID) to track all sources of consent, ensuring accurate status updates and full compliance.
- Different countries may require different robust consent processing methods, for example: two factor authentication, double opt-in confirmation emails or a digital signature applied on the consent document or even delegated consent processing to a ‘Consent Manager’. Using a dedicated consent management tool eliminates system fragmentation and enables these procedures to be deployed on a country-by-country basis, providing the most adequate local approach required.
- System access is also a crucial element; having the ability to employ unique username profiles with passwords, or tokenized access URLs, that are shared individually is key for data privacy.
- Updating consent and preferences, opt-outs or unsubscribing should be as simple and accessible as giving the original consent, and should be independent of the need to be in the presence of a pharma employee (field user).
- A permanent audit history of consent signing, the date and time, the user who processed the consent, the source system that provided the consent update with any subsequent changes logged, provides full confidence for both parties, life science organization and HCP.
- Finally, any possible regulatory changes and new requirements can be a challenge to incorporate, so having a dedicated consent management solution can ensure requirements are catered for on a country-by-country basis through an out-of-the-box approach.
Streamlining HCP consent
In HCP consent management, organizations face challenges like fragmented processes and data integrity concerns, which become even more challenging in multinational organizations. A unified approach with a consent master ensures compliance and direct HCP access to consent and preference centers builds trust.
Understanding consent management maturity across all markets is crucial for success. For a consent management project, a key recommendation is to assess the key components of policies, processes, training, and technology. Setting up senior management governance and sponsorship, with a clear definition of the business rules between functional areas such as medical, commercial, legal, etc., as well as different business units is a critical success factor.
If you would like help with an assessment or would like to learn more about IQVIA Consent Management technology, visit iqvia.com/hcpconsent.
References:
Navigating HCP Consent: Legislative Updates & Effective Practices. July 3, 2024.
Related solutions
Keeping up with evolving disclosure requirements is a challenge. Discover how IQVIA Transparency Reporting can help you adeptly report, global HCP spend.


























