Patient advocacy groups (PAGs) are formally organized nonprofit organizations that assist patients and/or caregivers and families of patients affected by medical conditions, and they work to ensure that patients receive timely and optimal care, increase awareness, improve understanding, and provide access to adequate support resources. There are about 3,327 patient organizations currently operating in the US, with approximately 18% focusing on rare diseases. Over the past 5 years, patient organizations have received over ~63 billion in revenue in the US, with an aggregate increase of 26% [1].
For biotech and pharmaceutical companies navigating the complexities of rare disease drug development, PAGs have emerged as indispensable partners. By strategically collaborating with PAGs, pharmaceutical companies can connect with their patient community, overcome key translational roadblocks, and accelerate clinical and commercial success through positioning clinical trials as viable options for rare disease patients, where approximately 95% of rare disease diagnoses still have no specifically approved treatments available.

One of the biggest obstacles in rare disease trials is the small, geographically dispersed patient populations with no or limited numbers of expert centers and/or experienced clinicians. Advocacy groups provide an unparalleled resource by directly connecting drug developers to potentially eligible patients through their comprehensive registries and community networks. Beyond recruitment, these groups lend critical insights that enhance study operations and designs. Leveraging the lived expertise of patients and caregivers, advocacy groups advise on patient-centric protocol elements like visit schedules, differences in standards of care, ways to reduce patient and caregiver burden, meaningful endpoints, and quality of life assessments [2].
On the commercial front, advocacy groups illuminate the comprehensive patient journey and demand landscape - informing everything from market sizing and segmentation to product positioning and service design. As companies plan market entry, advocacy groups can elevate disease awareness through their education platforms and provider networks. When it comes to market access, advocacy groups serve as authoritative expert voices supporting innovators throughout payer negotiations, formulary reviews, and regulatory processes. Thus, advocacy groups keep the patient voice resounding through every stage of the product lifecycle. Their continuous real-world data collection and community insights fuel iterative refinement of clinical programs, commercial models, and product support services [3][4].
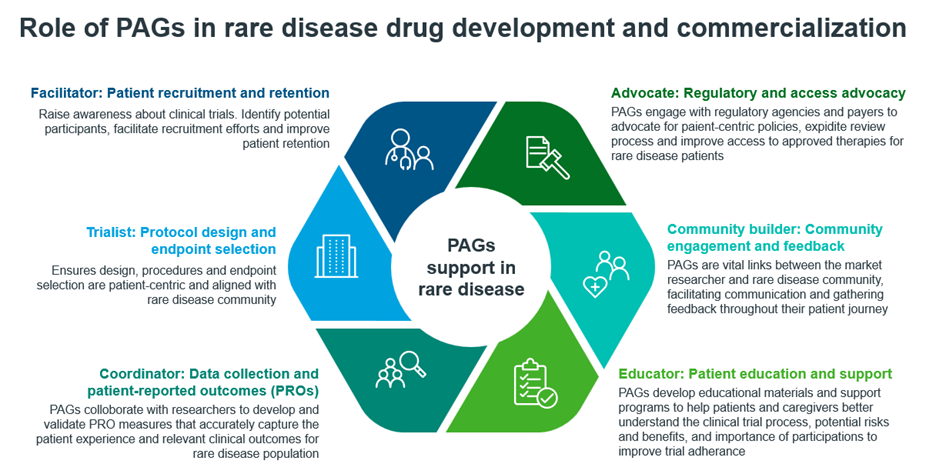
Catalyzing Clinical Development
- Patient Recruitment and Engagement: One of the biggest hurdles in rare disease clinical trials is finding and enrolling eligible participants from extremely small patient populations spread across the globe. Advocacy groups maintain rare disease patient registries and databanks that allow them to precisely pinpoint potential trial candidates. Their direct connections within the patient community enable effective grassroots awareness campaigns and referrals into trials [5]. Groups also provide peer mentors who educate patients about the clinical trial process, which improves retention and adherence to protocols [6][7].
- Patient-Centric Trial Design: By deeply understanding how rare diseases impact daily living, advocacy groups offer researchers a patient lens on prioritizing therapeutic areas, defining clinically meaningful outcomes, and optimizing study procedures. Their input ensures trials capture data that genuinely moves the needle for patients [8][9].
- Natural History Studies: Many advocacy groups sponsor natural history studies that systematically track how rare diseases progress over time. This real-world evidence is critical for contextualizing clinical data and designing trials with appropriate control arms [6][10].
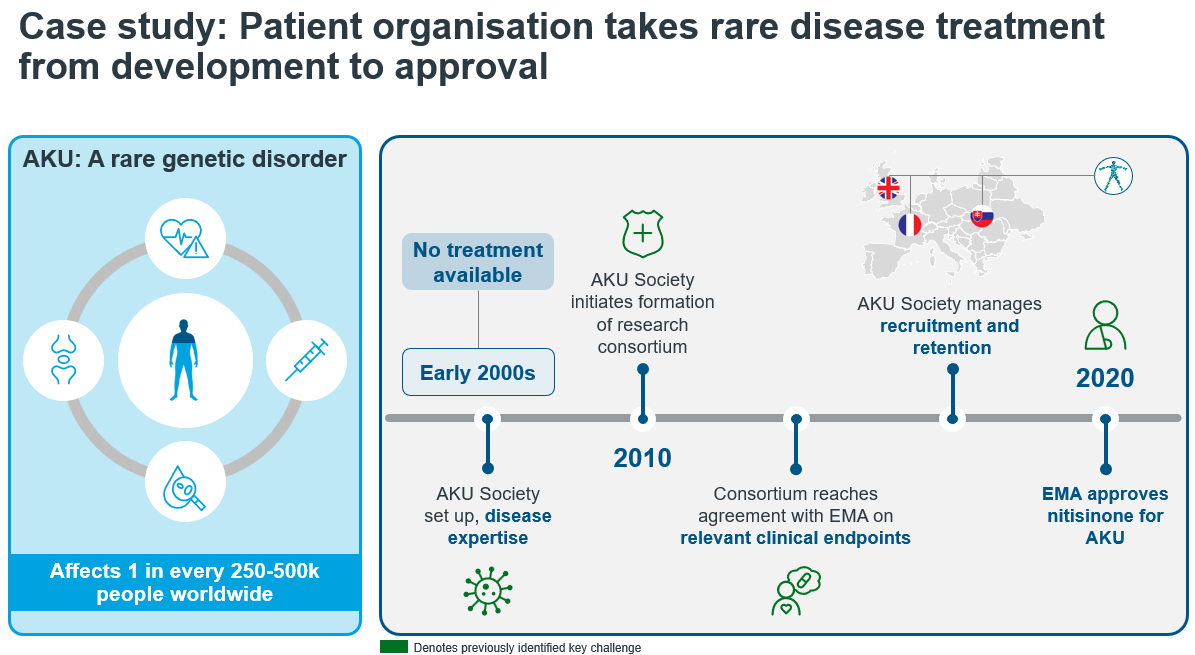
Source: IQVIA Whitepaper: Harnessing the Power of Patient Organizations: A Case Study of PO-Driven Drug Development in Ultra-Rare Disease. Accessed on June 2024. Available from: https://www.iqvia.com/-/media/iqvia/pdfs/library/white-papers/harnessing-the-power-of-patient-organisations.pdf [11]
Opening Commercial Pathways
- Market Intelligence: Advocacy groups gather granular demographic, epidemiological, and patient-reported data that delivers unmatched market insights. Their deep knowledge of factors like treatment journeys, economic impacts, and quality of life effects is invaluable for biotech companies to understand target patient populations and position therapies effectively.
- Regulatory consultation: Regulators like FDA and EMA regularly consult PAGs to review the process for rare therapies. PAGs provide insights into patient experience, help access risk-benefit profiles and highlight unmet needs. Beyond regulators, PAGs are also engaging with Health Technology Assessment (HTA) bodies to provide evidence on treatment value thus impacting reimbursement decisions [1].
- Disease Education: Advocacy groups raise public awareness of rare conditions through media campaigns, educational materials, screening programs, and conferences [5]. Their voices build understanding among physicians, payers, and policymakers and help create receptiveness to novel therapies when they launch.
- Patient Support Services: PAGs play multifaceted role in patient support programs (PSPs) for rare diseases: Designing PSPs in collaboration with pharma companies, developing and disseminating tailored educational material, care coordination with network of specialists, financial, and non-financial (adherence, training) assistance.
- Distribution Channels: Some prominent advocacy groups operate treatment centers and specialty pharmacies or have buy-and-bill programs (that purchase therapies upfront and then provide them to patients treated at participating physician practices and hospitals) for physician practices. These channels help ensure reliable, consistent product distribution for rare disease therapies [1].
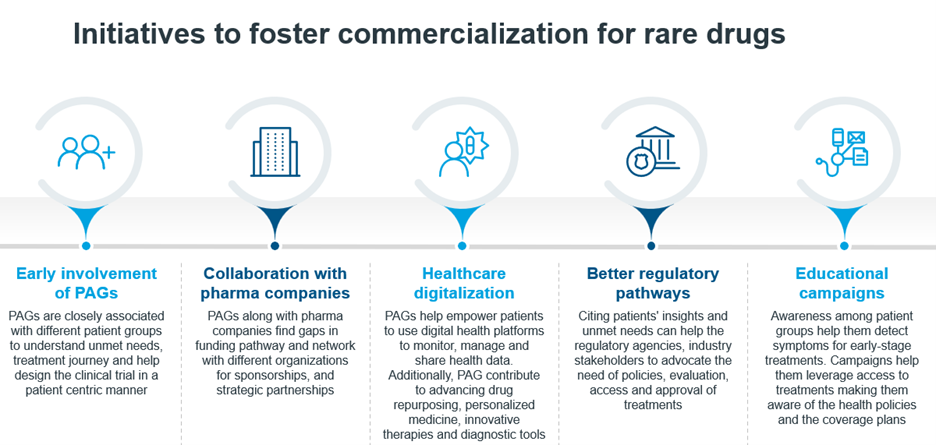
Where can IQVIA help our customers in clinical and commercial development?
The Pediatric and Rare Diseases Center of Excellence leverages medical expertise in pediatric & rare disease populations to align with diverse patient pathways, best practices, and lessons learned for strategizing and operationalizing clinical trials. On the other hand, IQVIA’s Research and Insights team, in collaboration with PAGs can uncover deep patient insights through ethnographic research, focus groups and surveys to understand the lived experiences, preferences, and challenges faced by rare disease patients and caregivers. These insights can inform product development, messaging and developing PSPs tailored to specific needs of the community. Here are some keys ways in which this collaboration can work:
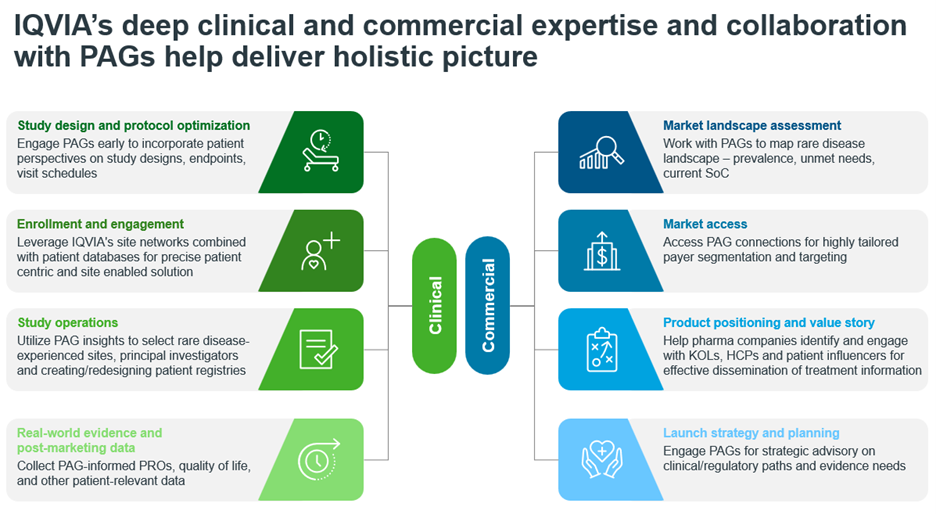
By integrating rich PAG insights across its portfolio of clinical and commercialization services, IQVIA positions itself as a strategic partner helping pharmaceutical companies optimize market readiness, market access, and product uptake for rare disease innovations to bring meaningful engagement and treatments to the patient and caregiver community represented by the PAGs.
References:
- Supporting patients through research collaboration. Source data from: Form 990 data from IRS Statistics of Income program. Years filed 2016-21. Accessed June 2024. Available from: https://www.iqvia.com/insights/the-iqvia-institute/reports-and-publications/reports/supporting-patients-through-research-collaboration
- FDA Center for Drug Evaluation and Research and Johns Hopkins Center of Excellence in Regulatory Science and Innovation (CERSI) Workshop. Accessed June 2024. Available from: https://publichealth.jhu.edu/sites/default/files/2023-09/rare-disease-day-1-slides-05022023.pdf
- The Global Role of Patients, Advocates and Caregivers in Rare Diseases. Accessed June 2024. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8131174/
- The involvement of rare disease patient organizations in therapeutic innovation across rare paediatric neurological conditions: a narrative review. Accessed June 2024. Available from: https://ojrd.biomedcentral.com/articles/10.1186/s13023-022-02317-6
- Recent advancement in the rare disease landscape. Accessed June 2024. Available from: https://www.iqvia.com/locations/united-kingdom/blogs/2023/02/recent-advancements-in-the-rare-disease-landscape
- Emerging roles and opportunities for rare disease patient advocacy groups Accessed May 2024. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10184204/
- The challenges of clinical trials in rare diseases. Accessed June 2024. Available from: https://onlinelibrary.wiley.com/doi/full/10.1111/bjd.21686
- Embedding Patient‑Centricity by Collaborating with Patients to Transform the Rare Disease Ecosystem. Accessed June 2024. Available from: https://link.springer.com/article/10.1007/s40290-023-00474-y
- Assessing Patient Preferences in Rare Diseases: Direct Preference Elicitation in the Rare Chronic Kidney Disease, Immunoglobulin A Nephropathy. Accessed June 2024. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8131174/
- Natural History and Real World Data in Rare Diseases: Applications, Limitations, and Future Perspectives. Accessed June 2024. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10107901/
- Harnessing the Power of Patient Organisations: A Case Study of PO-Driven Drug Development in Ultra-Rare Disease. Accessed June 2024. Available from: https://www.iqvia.com/-/media/iqvia/pdfs/library/white-papers/harnessing-the-power-of-patient-organisations.pdf





















